Inorganic Polymers
Properties
The vast majority of polymers are derived from oil, coal, gas, or (less frequently) from biomass such as plants, animals, or microorganisms
(fermentation), with carbon as the main or sole element in the polymer backbone. However, several other elements can also form (linear) polymer chains with properties similar or superior to those of conventional polymers.
A well known example are polysiloxanes ('silicones') which are used
in many industries for numerous applications.
A polymer is called inorganic if its backbone is either made up exclusively of non-carbon elements, or, when carbon is present, if it is not bonded to hydrogen or organic side groups. Sometimes a broader definition is chosen, which allows for organic side groups as long as these groups are not bonded to carbon in the main chain. Using the latter definition, a very large number of inorganic polymers exist which possess many advantageous properties.
The majority of linear inorganic polymers are derived from main-group elements such as silicon, phosphorus, sulfur, oxygen, nitrogen, boron, and carbon (not bonded to hydrogen or carbon in a side-chain). Carbon, sulfur, selenium and silicon can form long chain molecules on its own, whereas most other non-metal main group elements including nitrogen, phosphorous and oxygen form only linear chains when bonded to other elements. Examples for the former are polysilanes and polysulfides and for the latter are polysiloxanes and polyphosphates.
Inorganic repeat units in polymer backbones such as silicon-oxygen or phosphorus-nitrogen are typically much more thermo-oxidatively stable than their organic counterparts, and thus, possess superior heat- and fire-resistance. Some inorganic polymers may also provide some special properties such as electrical conductivity and photosensitivity. A major drawback of many non-carbon bonds is their sensitivity to hydrolytic cleavage. For example, linear silicates have limited stability to aqueous acids and bases. To improve their hydrolytic stability, organic side groups are often introduced which protect the polmyer backbone against hydrolytic attack. The organic side groups can also impart a greater degree of flexibility whereas the majority of fully inorganic polymers are rather brittle. Their low flexibility is the result of ionic bonds or a rigid three-dimensional covalent network.
Fully crosslinked inorganic polymers without organic side groups are known as ceramics. Ceramics such as silicon carbide, silicon nitride, aluminum oxide, and boron nitride are notorious for their extraordinary hardness, thermal stability, and high melting point. Since their properties are very different from plastic materials, they are not discussed further here.
Some of the most important linear inorganic polymers that can replace conventional plastics and polymers in many industrial and consumer applications are discussed below. The majority of these inorganic polymers have silicon, phosphorus, or sulfur atoms in the polymer backbone either as the sole element or bonded to carbon, nitrogen or oxygen.
Silicone
Polysiloxanes
Polysiloxanes are the most widely used inorganic polymers. They are also known as silicones and have found numerous applications across almost every industry. The name silicone usually refers to organosilicon polymers with the general structure -[Si(R2)-O]- where R = -CH3 is called poly(dimethyl siloxane) which is often abbreviated as PDMS:.
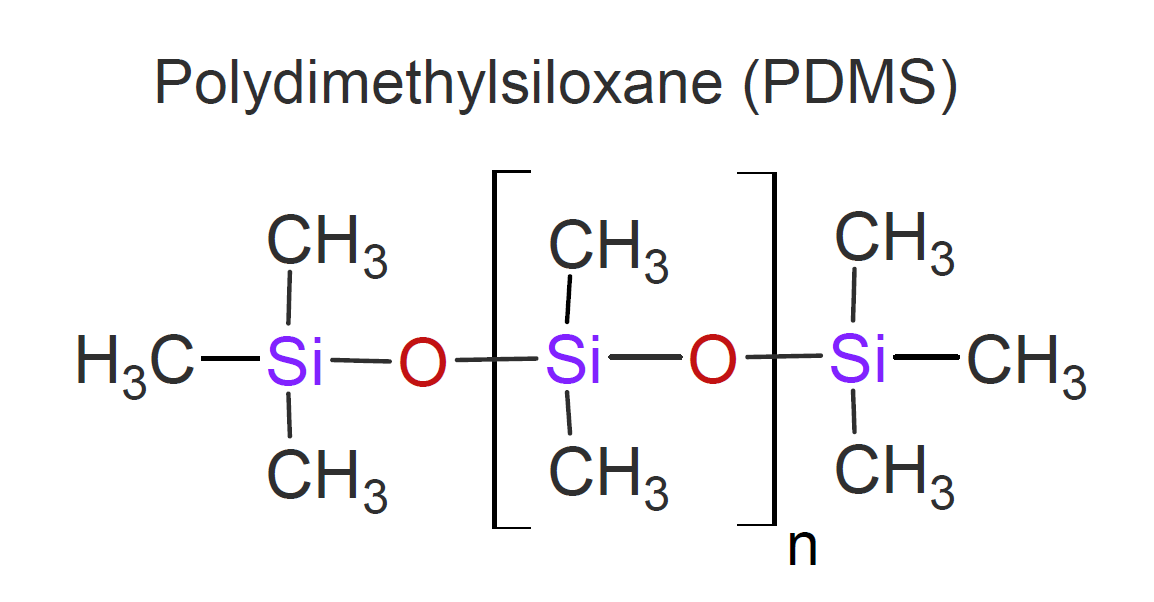
The backbone of this polymer is extremely resistant to skeletal oxidative cleavage at elevated temperatures because there is no oxidative driving force
since the bonds to each silicon
are already linked to oxygen. PDMS also possesses excellent low temperature flexibility, as well as outstanding resistance to weathering and many chemicals.
Many other groups like phenyl, vinyl, alkyl, or trifluoropropyl can substitute the methyl groups along the
polymer backbone which allows for a wide variation of properties including solubility, hydrophobicity or hydrophilicity, refractive index, and mechanical modulus.
Polysilanes
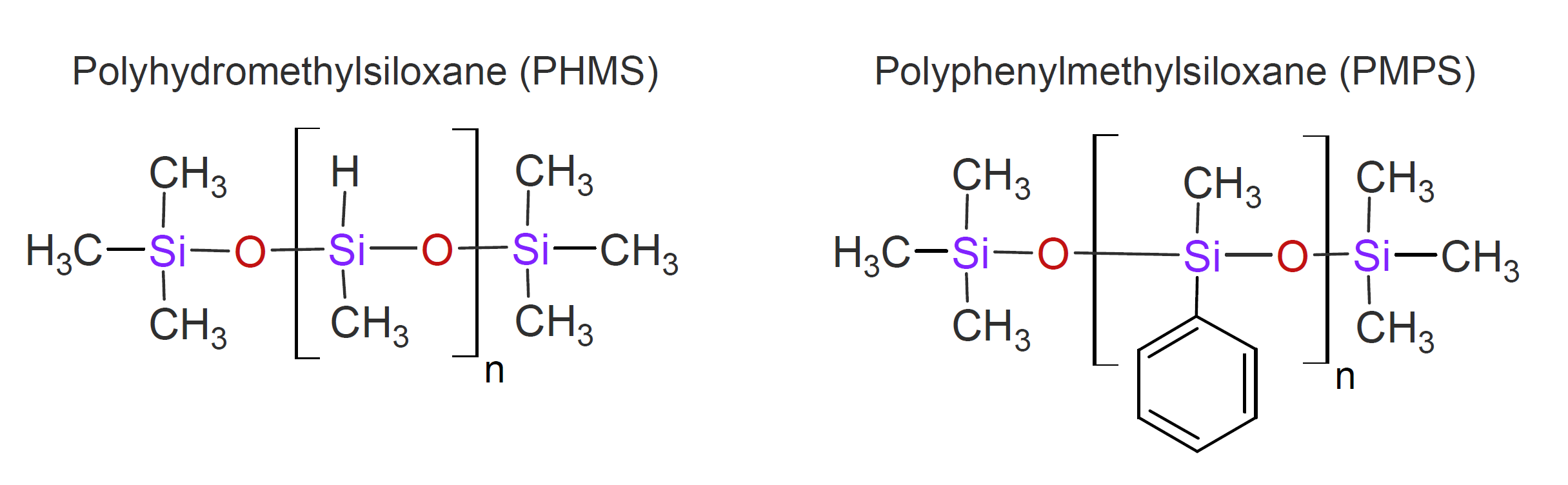
In polysilanes, the backbone of the polymer is made up entirely of silicon with two substituents attached to each silicon atom. Depending on the side groups, this class of inorganic polymers covers a wide range of properties from highly crystalline and rigid to fully amorphous and very flexible solids.
Most of the polysilanes have excellent heat resistance and are not very susceptible to hydrolysis but photodegrade when exposed to ultraviolet light. They also possess unique electronic properties resulting from the interaction between the relatively large adjacent silicon orbitals which causes delocalization of sigma electrons in the Si-Si bonds, i.e. sigma-electron delocalization.7 These materials could function as photoresists and as photoconductors in microelectronic devices.
Polysilazanes
Polysilazanes are a class of very heat stable inorganic polymers with a polymer backbone made up entirely of silicon-nitrogen bonds with either only hydrogen substituents attached to each silicon and nitrogen atom (perhydro-polysilazanes PHPS, -NH-SiH2-) or with additional organic substituents attached to each silicone (organo polysilazanes, OPSZ, -NH-SiR2-).
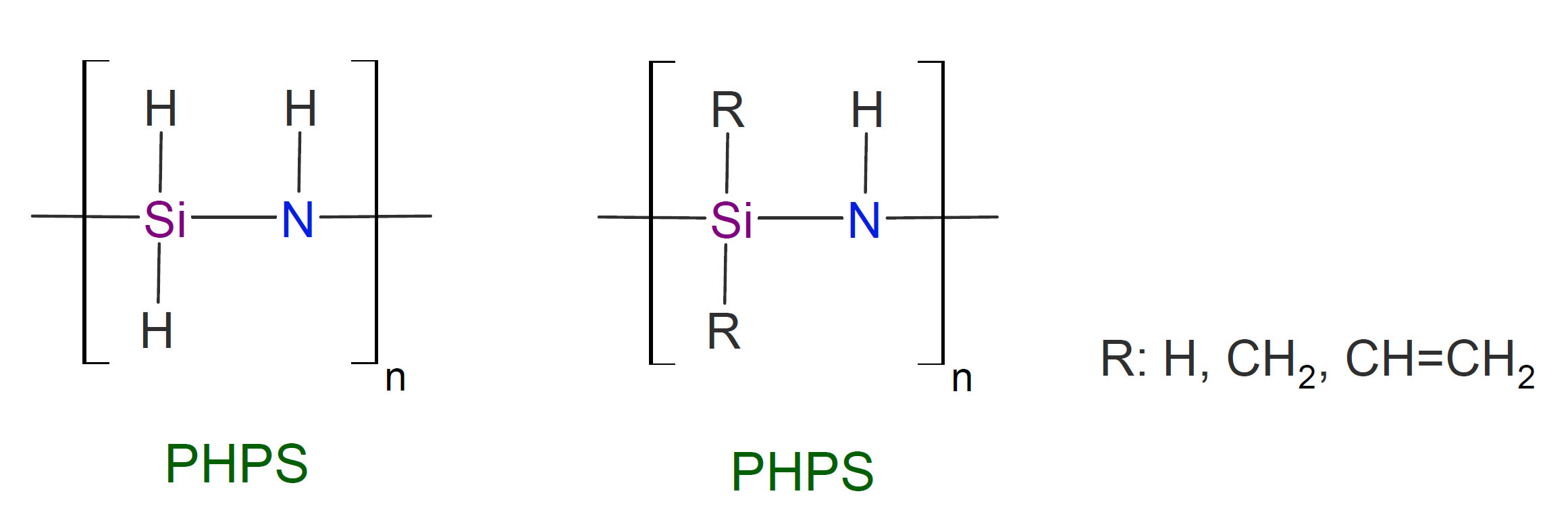
Polysilazanes are known for their high temperature and oxidative stability and excellent scratch, abrasion and impact resistance. They also possess high resistance to weathering and many chemicals. Most of their properties are superior to those of polysiloxanes. Polysilazanes with nonpolar organic side groups have also outstanding non-stick and easy clean properties which are almost as as good as those of fluoropolymers (PTFE).
Phosphorous
Polyphosphates
Polyphosphates (polyP) consist of linear polymers of orthophosphate units linked together by high-energy phosphate anhydride bonds. These polymers can be either completely inorganic if the counter ion is an alkali metal such as sodium, potassium or ammonium, or they can be semi-inorganic if some or of the oxygen is bonded to organic groups. The latter are known as polyphosphate esters.
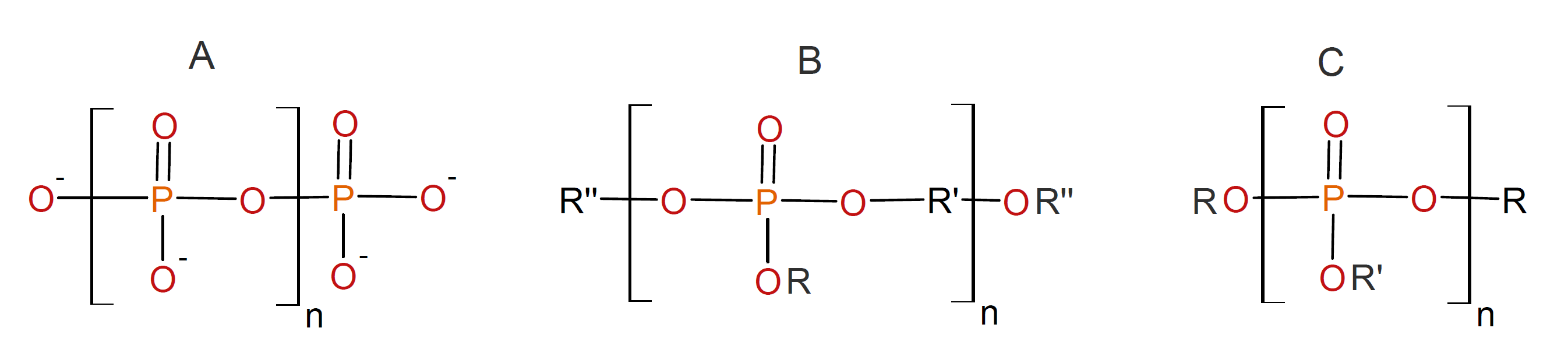
The inorganic polyphosphates are glass-like, water-soluble polymers that can have a linear, cyclic, or crosslinked structure. The short chain and cyclic sodium polyphosphates are of great importance as detergents but have been (partially) replaced in many products due to their negative environmental impact when discharged into surface water (eutrophication). Crosslinked polyphosphates are used as ion exchange resins. Some semi-aromatic polyphosphates have been synthesized from phosphorodichloridates with aromatic diols. These polymers have high transparency, hardness and flame retardancy. However, they also have poor long-term hydrolytic stability and, thus, have found only limited commercial use, mainly as novel plasticizers and flame retardants for PVC.
Polyphosphazenes
Polyphosphazenes are by far the largest class of inorganic polymers. Hundreds of different polymers of this type have been synthesized, with a range of physical and chemical properties that rival those of organic polymers.1,2 This class of polymers possesses many valued properties such as high thermal and chemical stability and comparatively low toxicity which makes them attractive materials for numerous biomedical, elastomeric, and electrochemical applications. The properties of this class of inorganic polymers can be varied over a wide range by changing the type of side groups, the molecular weight, and cross-link density. Some forms of unmodified and modified polyphosphazenes are depicted below.
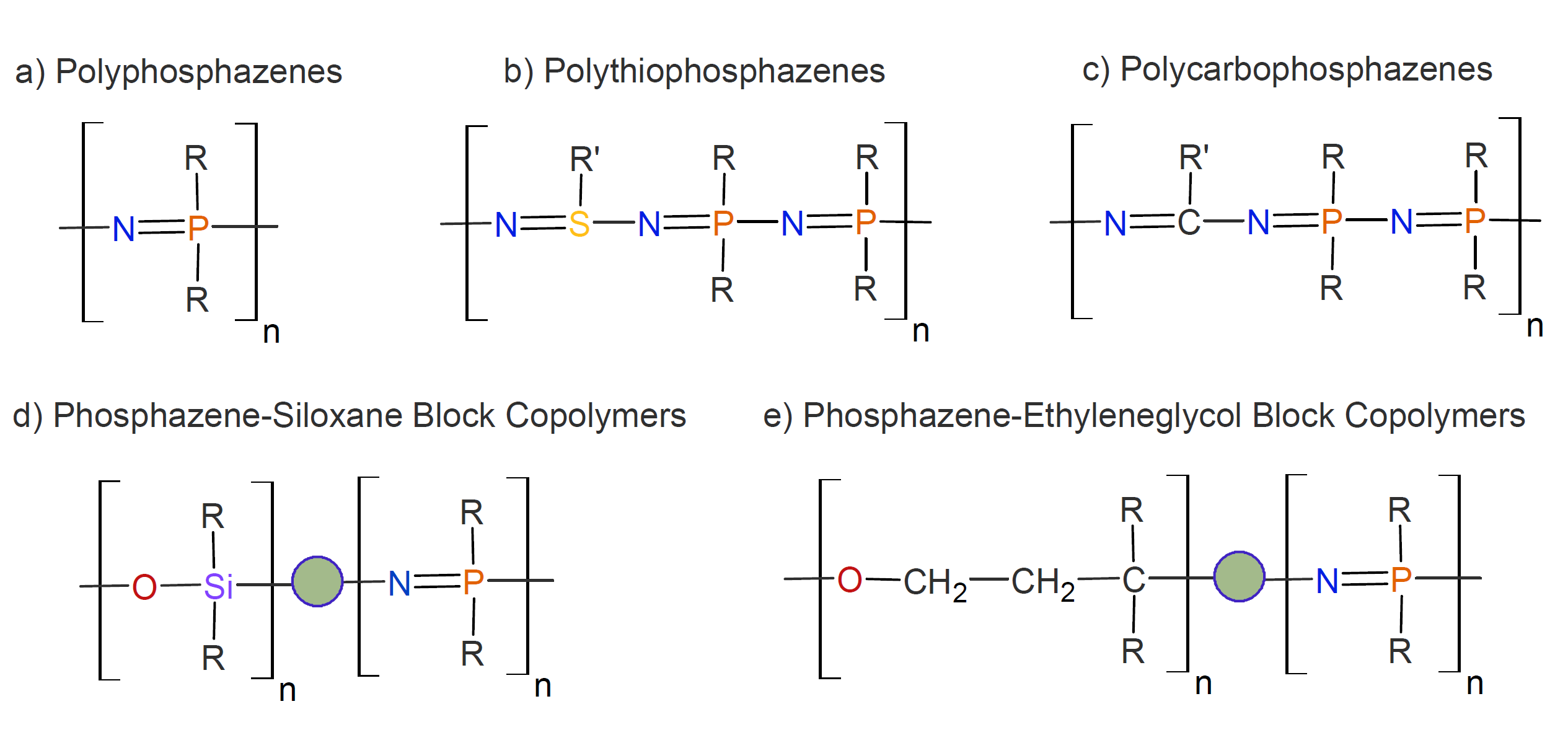
The polymer backbone of unmodified polyphosphazene consists of alternating phosphorus and nitrogen atoms, with two side groups, R, being attached to each phosphorus (a). These side groups may be organic, organometallic, or inorganic. Also obtainable are polymers in which a portion of the phosphorus atoms has been replaced by sulfur (b) or carbon atoms (c) or in which polyphosphazenes are linked to other polymers such as polysiloxanes (d) or polyethers (e).
Sulfur
It is well known that sulfur vulcanizes diene elastomers such as polyisoprene and polybutadiene where sulfur atoms form short chains that react with dienes in the rubber. Besides short bridges between rubber chains, sulfur has also the ability to form long polymer chains. The simplest sulfur-based polymer is prepared by ring-opening polymerization of rhombic sulfur, which is made up of eight-membered sulfur rings. This polymer forms at temperatures above 159°C, but above approximately 175°C it spontaneously depolymerizes.1,8 The resulting polymer is highly elastomeric above its glass-transition temperature of approximately -30°C but is unstable at room temperature and gradually crystallizes, and eventually reverts entirely back to cyclic rhombic sulfur (Backbiting).
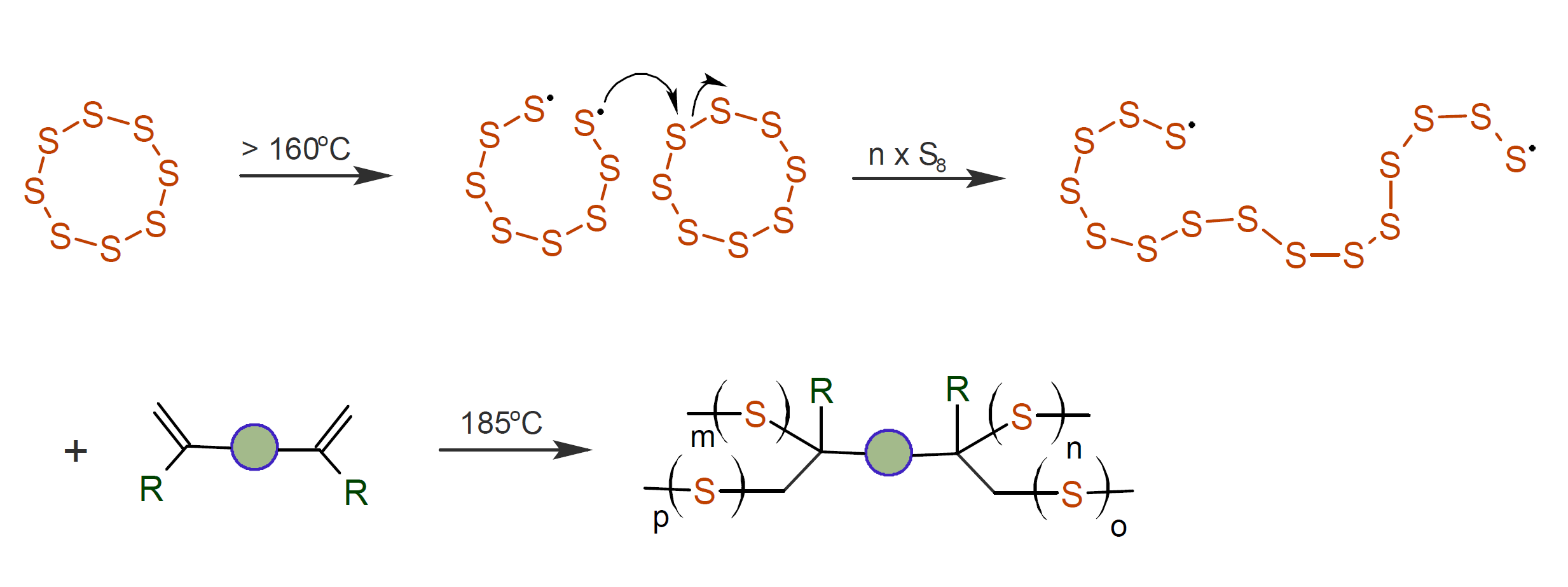
To prevent depolymerization, the thiyl radicals must be quenched during polymerization for example by crosslinking the linear polymeric sulfur with diene crosslinkers which results in branching and crosslinking of the polymeric chains (see figure above). Termination can be achieved in two ways: the thiyl radicals can either undergo intramolecular recombination and form stable polysulfide loops or, in cases H-atoms are available (alkene co-monomers), the thiyl radicals may abstract hydrogen from the donor molecule and convert to a thiol.8
Another sulfur-based polymer that has been successfully synthesized is poly(sulfur nitride) (also known as polythiazyl).1,7 This completely inorganic polymer can be obtained by sublimation of tetrasulfur tetranitride [SN]4, an eight-membered cyclic tetramer.1 This highly crystalline polymer behaves like a metal with an ambient electrical conductivity of same order of magnitude as metals. Unfortunately the polymer oxidizes easily, is insoluble and infusible, which prevents its practical use as a semiconductor.
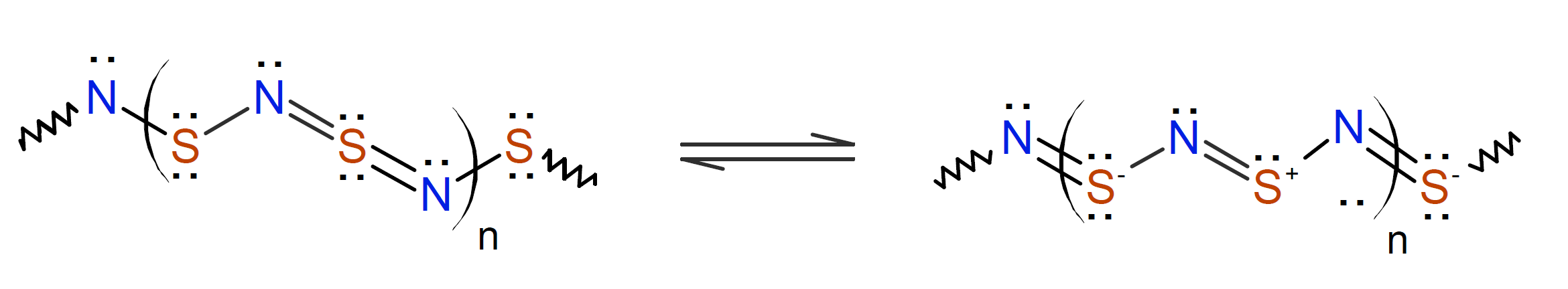
This polymer (inherent/doped) can be used as a conductive material in electronic devices and in electrically conductive plastics such as battery housings or head assemblies for light sources.5
Miscellaneous
Several other elements can replace carbon in the polymer backbone. However, most of these inorganic polymers have found little or no commercial use. Two such elements are germanium and tin which are directly below carbon and silicon in the periodic table. Similar to polysilanes, polymers of these elements can be synthesized by Wurtz-reductive-coupling which yields polymers with a structure very similar to that of polysilanes.7
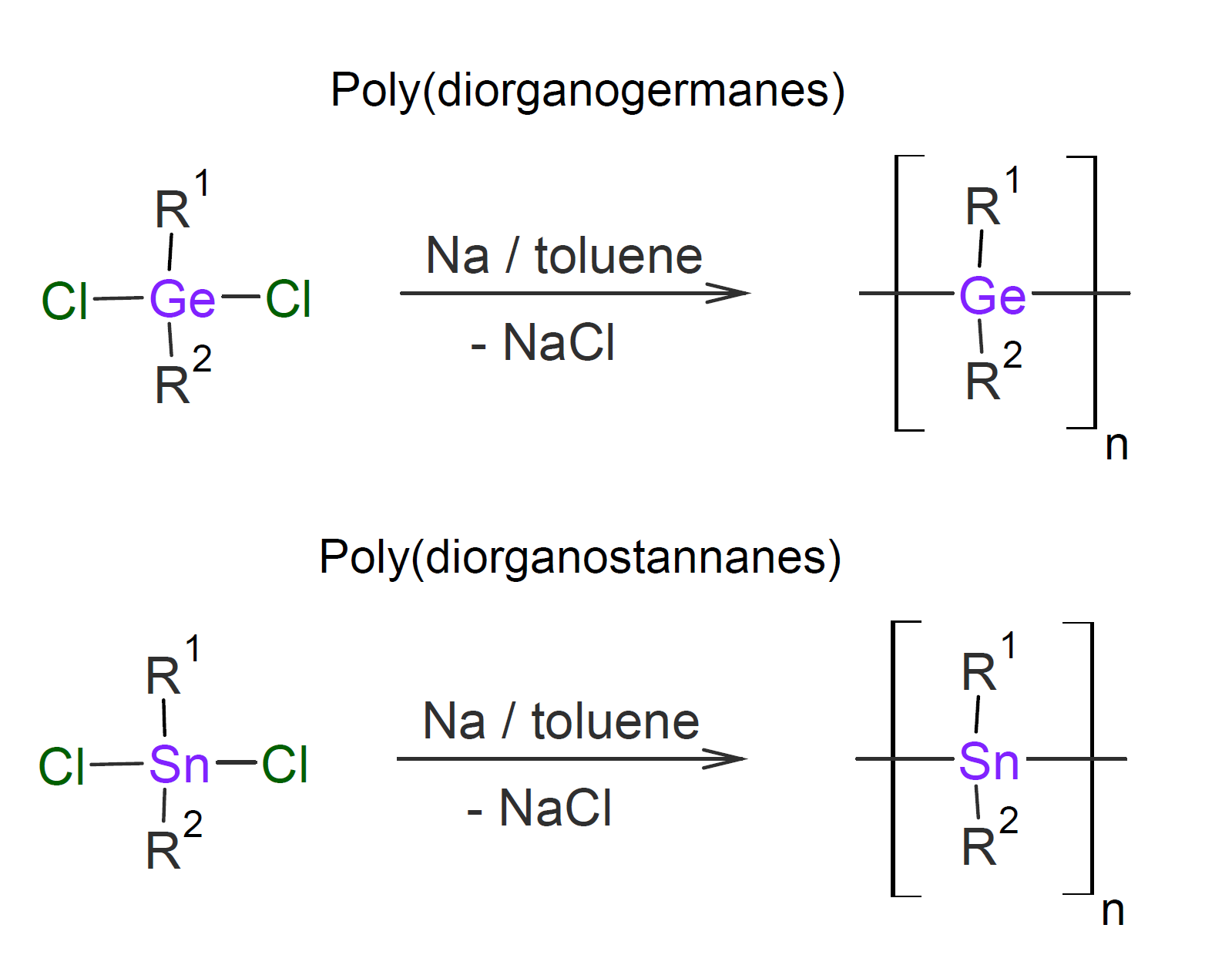
Both polygermanes and polystannanes are semiconducting materials due to inherent σ-delocalization and possess interesting optical and electronic properties, and therefore have been intensively studied.
A number of linear polymers containing boron have also been synthesized. One of the simplest boron containing polymers has been prepared by the reaction of elemental boron with boron trifluoride at high temperatures.1 This polymer is a rubbery elastomer that has not found any major uses due to its hydrolytic instability and high flammability. A number of other boron containing polymers have been prepared by heating borazine at slightly elevated temperatures (70C) in vacuo:
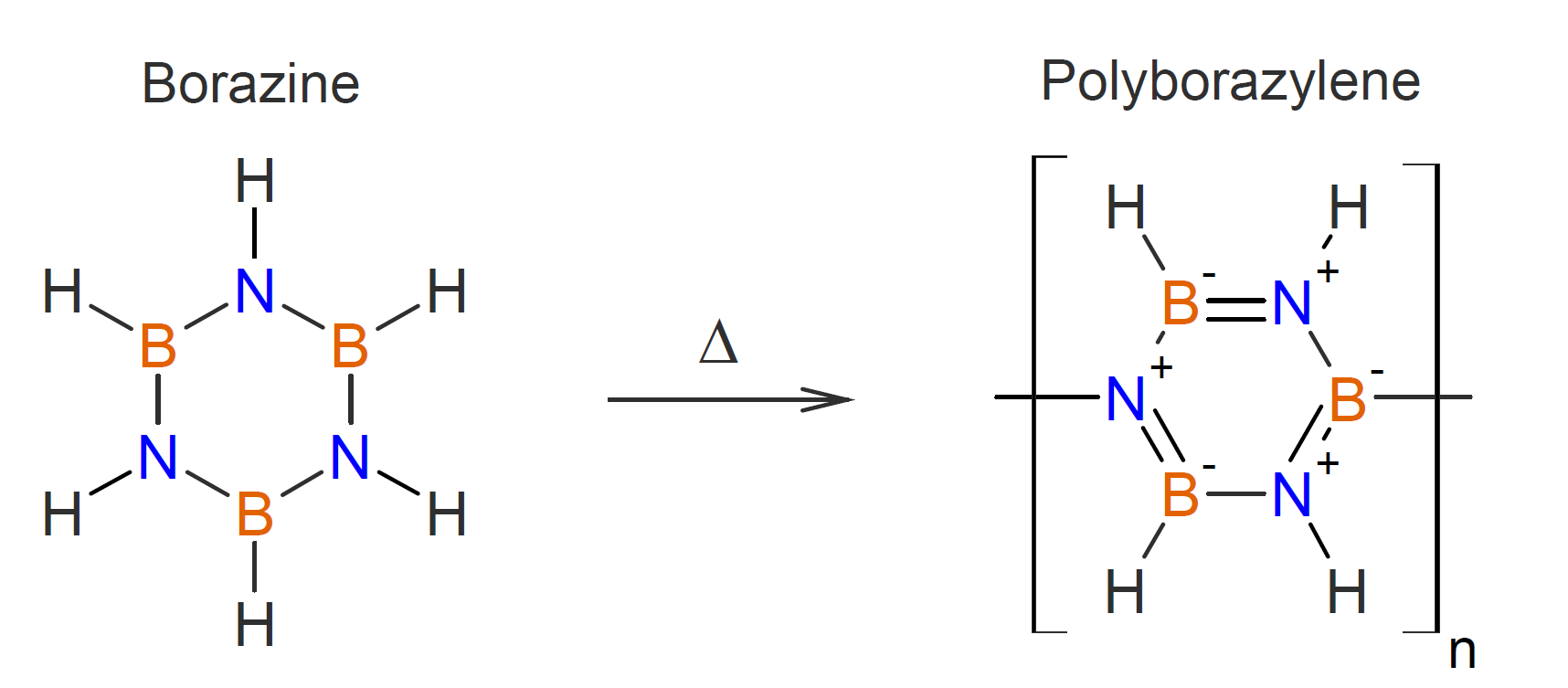
This polymer can be converted to boron nitride in high yield when pyrolyzed under argon or ammonia at temperatures between 900°C and 1450°C.9,10